Case Study Question 1 on Chemical Kinetics – Chapter 4
Read the given passages and answer the questions that follow.
Question. The rate of a chemical reaction is expressed either in terms of decrease in the concentration of a reactant per unit time or increase in the concentration of a product per unit time. Rate of the reaction depends upon the nature of reactants, concentration of reactants, temperature, presence of catalyst, surface area of the reactants and presence of light. Rate of reaction is directly related to the concentration of reactant. Rate law states that the rate of reaction depends upon the concentration terms on which the rate of reaction actually depends, as observed experimentally. The sum of powers of the concentration of the reactants in the rate law expression is called order of reaction while the number of reacting species taking part in an elementary reaction which must collide simultaneously in order to bring about a chemical reaction is called molecularity of the reaction.
1. Express the rate of the following reaction in terms of different reactants and products.
4NH3(g) + 5O2(g) —–> 4NO2(g) + 6H2O(g)
Ans.
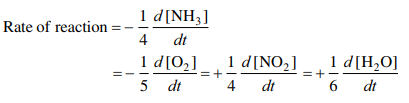
2. Why do pieces of wood burn faster than a log of wood of the same mass?
Ans. Pieces of wood have larger surface area than the log of wood of the same mass. Greater the surface area, faster is the reaction.
3. Why does the rate of any reaction generally decrease during the course of the reaction?
Ans. The rate of reaction depends on the concentration of reactants. As the reaction progresses, reactants start getting converted to products so the concentration of reactants decreases hence the rate of reaction decreases.
4. Why is molecularity applicable only for elementary reactions and order is applicable for elementary as well as complex reactions?
Ans. A complex reaction proceeds through several elementary reactions. Number of molecules involved in each elementary reaction may be different, i.e., the molecularity of each step may be different. Therefore, discussion of the molecularity of overall complex reaction is meaningless. On the other hand, order of a complex reaction is determined by the slowest step in its mechanism and is not meaningless even in the case of complex reactions.
5. The kinetics of the reaction
mA + nB + pC —–> m′X + n′Y + p′Z
obey the rate expression dx/dt = k[A] m[B] n
Calculate total order and molecularity of the reaction.
Ans. The total order of reaction = m + n
The molecularity of the reaction = m + n + p
