Chapter 1
Electric Charges and Fields
Topics Covered : Introduction, Polarity, Charge, Methods of charging
Introduction
Electrostatics is a branch of physics that studies electric charges at rest.
Electric charge like mass is a fundamental property associated with elementary particles like electrons, proton, neutrons, etc. We can say that electric charge is the physical property of matter because of which it experiences a force when it is placed in an electromagnetic field.
According to William Gilbert,
“The charge is something possessed by material objects that make it possible for them to exert electrical forces and to respond to electrical force.”
Polarity
The property which differentiates the two kinds of charges is called the polarity of charge.
In physics, polarity is a description of an attribute, typically a binary attribute (one with two values), or a vector (a direction). For example:
- An electric charge has a polarity of either positive or negative.
- A battery contains polarity, with the two + and – terminals. Similar to electric charge, the energy flows from the positive terminal, through the battery, to the negative terminal, and exits the dry cell.
- A voltage has a polarity, in that it could be positive or negative (with respect to some other voltage, such as the one at the other end of a battery or electric circuit).
- A magnet has a polarity, in that one end is the “north” and the other is the “south”.
A particle, atom, or object with negative charge is said to have negative electric polarity; a particle, atom, or object with positive charge is said to have positive electric polarity.
If a body possess an electric charge, it is said to be charged or electrified. When it has no charge, it is said to be neutral.
The vast amount of charges is hidden as the object contains equal amounts of positive and negative charge. With such an equality or balance of charge, the object is said to be electrically neutral, i.e., it contains no net charge.
If the charges in object are not balanced, then there is a net charge and hence object charged.
When we say that a body is charged we always refer to excess charge or deficit charge. (Refers to loss or gain of electrons)
Body which is having lower work function will lose the electrons and vice versa.
Work function refers to whether electrons are held more tightly or held less tightly.
What is charge made of? Or how is the electric charge produced?
Electric charges cannot be produced or destroyed. We can, however, create or destroy particles that carry an electric charge. Total charge or net charge always remains conserved. As mentioned earlier transfer of charge from one body to another results in that body becoming electrically charged (either positive or negative).
Origin of an electric charge?
Electric charge originates due to the transfer of electrons from one material to another. This transfer of electrons leaves atoms of matter with unequal numbers of electrons and protons. Transfer of electrons from one material to another takes place by rubbing two objects against each other. If there are less number of electrons in an object, then the object is positively charged. If there are more electrons, then the object is negatively charged.
What are the 3 methods of charging?
The three methods of charging are
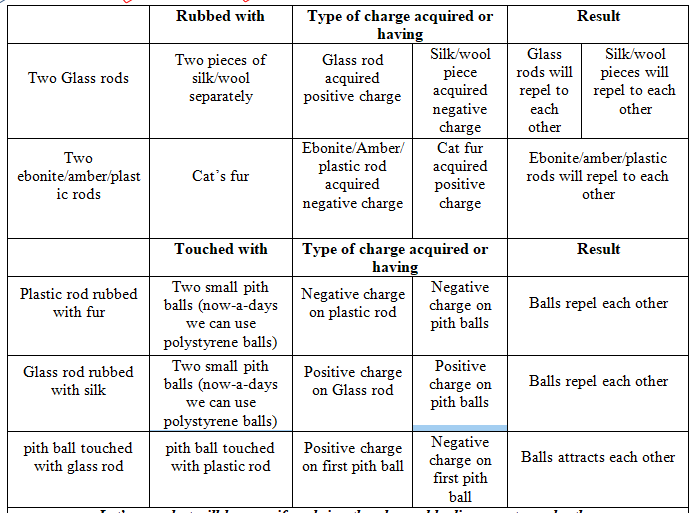
Charging by rubbing:
The simplest way to experience electric charge is to rub certain bodies among each other. Rubbing or friction makes electrons move. This gives one material a positive charge and the other a negative charge.
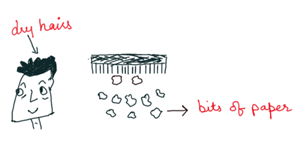
If we run a comb through our hair, the comb gets charged and can attract tiny pieces of paper. This is because the comb may have lost its electrons or gained some electrons when we rub it with the scalp. This comb is now a charged body. The net charge on the comb interacts with the net charge on small pieces of paper resulting in attraction. Many such solid materials are known to attract light objects like light feathers, pieces of paper, straw, etc.
Take another example of a glass rod and a silk fabric. Now if we rub a glass rod with a silk cloth, the silk cloth absorbs electrons from the glass rod and gets negatively charged. As a consequence, the glass rod is positively charged due to the loss of electrons.
In this case, bar after rubbing, comb after running through dry hair becomes electrified and this is an example of frictional electricity.
It is important to remember here that only non-metallic bodies can be charged by friction. The explanation is that the charges are free to travel within the material in a metal or conductor. If you apply a charge to one component of the conductive material, the other charges will be automatically re-arranged to neutralize the charge.
In the case of non-metallic or non-conductors, the charges you put on the surface of the material by rubbing remain as there is no re-arrangement taking place. This is because the insulator charges do not pass freely inside the content.

Conclusion:
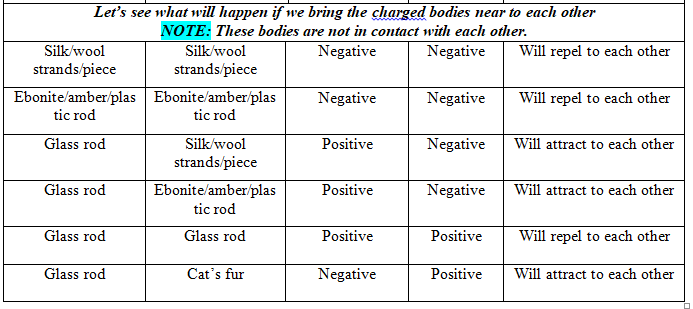
Charge acquired by the objects on rubbing against each other must be equal and opposite.
Like charges attracts to each other and opposite charges attracts to each other.
Note:
Du Fay was the first to show two kinds of charges.
Benjamin Franklin an American scientist named the two kinds of charges as positive and negative.
Charging by induction:
Induction charging is a method used to charge an object without actually touching the object to any other charged object.
Or
When a body is charged by induction then there is no transfer of electrons from one body to another. In charging by induction there is no physical contact between the charging body and the conductor being charged.
Charging a Two-Sphere System Using a Positively Charged Object
Bring two metal spheres, A and B, supported on insulating stands, in contact with each other.
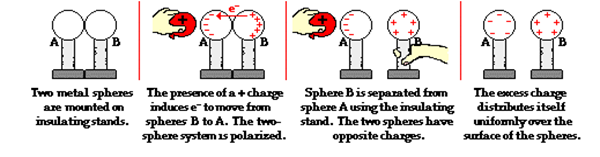
Bring a positively charged sphere near one of the spheres; say A, taking care that it does not touch the sphere. The free electrons in the spheres are attracted towards the rod.
The left surface of sphere A, has an excess of negative charge and the right surface of sphere B, has an excess of positive charge. However, not all of the electrons in the spheres have accumulated on the left surface of A. As the negative charge starts building up at the left surface of A, other electrons are repelled by these.
Separate the spheres by a small distance while the positively charged sphere is still held near sphere A. The two spheres are found to be oppositely charged and attract each other.`
Remove the rod positively charged sphere. The charges on spheres rearrange themselves. Now, separate the spheres quite apart. The charges on them get uniformly distributed over them.
Charging by conduction:
When we bring two conductors, one charged and other uncharged in contact, the same type of charge will appear on both the conductors.
