Chapter 1
Electric Charges and Fields
Topics Covered : COULOMB’S law, Limitations of Coulomb’s Law, Coulomb’s law in vector form, Dielectric constant or relative electrical permittivity
COULOMB’S law
Charles Coulomb observed that when two electric charges are placed close to each other, they experience a force. He used a torsion balance to measure the repulsive and attractive forces between charged particles.
Statement of Coulomb’s law
First law
Coulomb’s first law states that two charged particles of same charge (positive or negative) will repel each other and two charged particles of opposite charges (one positive and one negative) will attract each other.
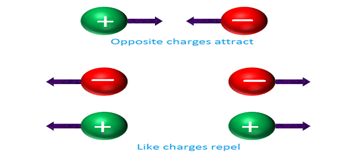
In other words, if two positively or negatively charged particles are placed close to each other, they get repelled. On the other hand, if one positively charged particle and one negatively charged particle is placed close to each other, they get attracted.
Second law
Coulomb’s second law states that, the force of attraction or repulsion between the two electrically charged particles (q1 & q2) is directly proportional to the product of magnitudes of two charges and inversely proportional to the square of the distance between two charges.
This force of attraction or repulsion between two charges is also depends on the medium in which charges are placed.
If we increase the distance between two point charges, the force of attraction or repulsion present between them will decrease. In the similar way, if we decrease the distance between two point charges, the force of attraction or repulsion present between them will increase.
Coulomb’s law can be mathematically written as
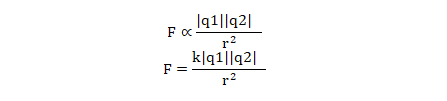
Where,
F = Force of attraction or repulsion between the charges
q1, q2= Magnitude of charge 1 and charge 2
r = Distance between two charges.
k = Constant whose value depends on the medium in which charges are placed.
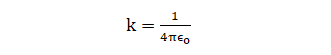
Where,
ε0 = absolute electrical permittivity of vacuum/free space
Value of k in SI is 9 x 109 Nm2C-2 and in cgs system is k=1
Units, Dimensions and Value of ε0
Unit: C2N-1m-2
Dimension: M-1L-3T4A2
Value: In SI unit; ε0 = 8.854 x 10-12 Nm2C-2
In CGS unit; ε0=1
Limitations of Coulomb’s Law
- It is not applicable to moving charges i.e. Coulomb’s Law is apply on static charges and charge must be stationary with respect to each other.
- It is applicable to charges of regular and smooth shape. It is very difficult to apply on irregular shape.
- The charges must not overlap for example they must be point charges.
- This charge cannot be directly applicable to calculate the charge on big planets.
Coulomb’s law in vector form

From Equation (1) & (2) we can conclude that force on q1 due to q2 is equal and opposite to the force on q2 due to q1. i.e.,
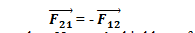
Hence, we can say Coulomb’s law obey Newton’s third law of motion.
NOTE:
- Coulomb’s law of electrostatic force between two charges is corresponds to the Newton’s law of gravitational force between two masses.
- Electrostatic forces are stronger than the gravitational forces. Example, A charged glass rod attracts the piece of papers against the gravitational pull of earth on the paper.
Dielectric constant or relative electrical permittivity
When the charges are placed in a medium other than free space (vacuum or air), the force between is given by
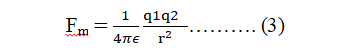
Where is called the absolute electrical permittivity of the intervening medium.
When the charges are placed in free space (vacuum or air), the force between is given by

Dividing (4) by (3), we get
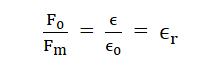
Where is called relative electrical permittivity of the medium. It is also called the dielectric constant of the medium and is denoted by K.
Thus may define:
The ratio of the absolute electrical permittivity of the substance/medium to the absolute electrical permittivity of the free space.
Or,
The ratio of force of interaction between two point charges separated by a certain distance in air/vacuum to the force of attraction/repulsion between the same two point charges, held the same distance apart in the medium.
NOTE:
- A dielectric is a material which has poor electrical conductivity but inherits an ability to store an electrical charge (due to Dielectric polarization).
- It is a unitless, dimensionless quantity.
- Dielectric constant expresses the extent to which a material can hold electric flux in it and its value depends only on the nature of medium.
For Example
| Medium | Value of K |
| Vacuum | 1.000000 |
| Air | 1.006 |
| Hydrogen | 1.00026 |
| Glass | 3 to 4 |
| Mica | 3 to 6 |
| Water | 81 |
