Solutions – Chapter 2 CBSE Class 12 Chemistry
Short Answer Questions – 2 marks
Q. 1. State Henry’s law. Write its one application. What is the effect of temperature on solubility of gases in liquid? [CBSE (F) 2016]
Ans. It states that the partial pressure of a gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution.
p ∝ x or p = KH x where KH is the Henry’s constant.
Application of Henry’s law:
To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
Effect of temperature on solubility:
As dissolution is an exothermic process, therefore, according to Le Chatelier’s principle solubility should decrease with rise in temperature.
Q. 2. Henry’s law constant (KH) for the solution of methane in benzene at 298 K is 4.27 ×105
mm Hg. Calculate the solubility of methane in benzene at 298 K under 760 mm Hg. [CBSE (F) 2013]
Ans.
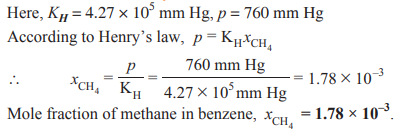
Q. 3. State Raoult’s law for the solution containing volatile components. What is the similarity between Raoult’s law and Henry’s law? [CBSE Delhi 2014; 2020 (56/5/1)]
Ans. It states that for a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction. According to Raoult’s law, for a volatile component, A of the solution PA ∝ xA or PA = P0A xA , where P0A is the vapour pressure of pure component A.
If one of the component is so volatile that it exist as a gas then according to Henry’s law p = KHx, where
KH is the Henry law constant i.e., the partial vapour pressure of the volatile component (gas) is directly
proportional to its mole fraction in the solution.
Thus the similarity between Raoult’s law and Henry’s law is that in both the laws, the partial vapour
pressure of the volatile component or gas is directly proportional to its mole fraction in the solution.
Q. 4. State the following:
(i) Raoult’s law in its general form in reference to solutions.
(ii) Henry’s law about partial pressure of a gas in a mixture. [CBSE (AI) 2011]
Ans. (i) Raoult’s law: It states that for any solution, the partial pressure of each volatile component in the
solution is directly proportional to its mole fraction.
(ii) Henry’s law: It states that the partial pressure of a gas in vapour phase (P) is proportional to its mole
fraction (x) in the solution.
Q. 5. Define an ideal solution and write one of its characteristics. [CBSE Delhi 2014]
Ans. A solution which obeys Raoult’s law over the entire range of concentration is called ideal solution. The important characteristics of an ideal solution are
(i) The enthalpy of mixing of pure components to form the solution is zero i.e., ∆mixH = 0
(ii) The volume of mixing is zero i.e., ∆mixV = 0
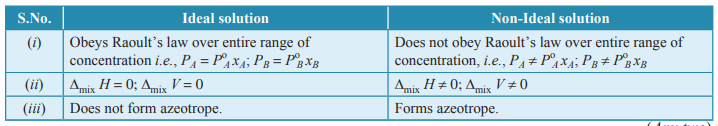
Q. 6. State Raoult’s law for the solution containing volatile components. Write two differences between an ideal solution and a non-ideal solution. [CBSE 2015]
Ans. Raoult’s law states that for a solution of volatile liquids the partial vapour pressure of each component is directly proportional to its mole fraction.
Differences between Ideal and non-Ideal solutions
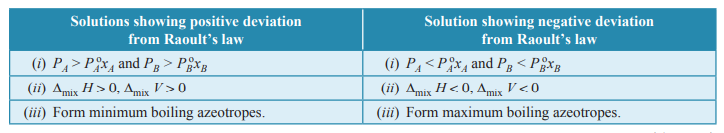
Q. 7. Write two differences between a solution showing positive deviation and a solution showing negative deviation from Raoult’s law. [CBSE 2016]
Ans.
Q. 8. (i) Why is an increase in temperature observed on mixing chloroform and acetone? [CBSE 2019 (56/2/3)]
(ii) Why does sodium chloride solution freeze at a lower temperature than water? [CBSE (F) 2013]
Ans. (i) The bonds between chloroform molecules and molecules of acetone are dipole-dipole interactions
but on mixing, the chloroform and acetone molecules, they start forming hydrogen bonds which are
stronger bonds resulting in the release of energy. This gives rise to an increase in temperature.
(ii) When a non-volatile solute is dissolved in a solvent, the vapour pressure decreases. As a result, the
solvent freezes at a lower temperature.
Q. 9. Define azeotropes. What type of azeotrope is formed by negative deviation from Raoult’s law? Give an example. [CBSE Delhi 2015]
Ans. Azeotropes are binary liquid mixtures having the same composition in liquid and vapour phase and boil at a constant temperature.
Maximum boiling azeotrope is formed by negative deviation from Raoult’s law. A mixture of 68% nitric
acid and 32% water by mass is an example of maximum boiling azeotrope.
Q. 10. What type of deviation is shown by a mixture of ethanol and acetone? What type of azeotrope is formed by mixing ethanol and acetone? [CBSE (F) 2013]
Ans. A mixture of ethanol and acetone shows positive deviation and the azeotrope formed by this mixture is minimum boiling azeotrope.
Q. 11. (i) Gas (A) is more soluble in water than Gas (B) at the same temperature. Which one of the two gases will have the higher value of KH (Henry’s constant) and why?
(ii) In non-ideal solution, what type of deviation shows the formation of maximum boiling azeotropes? [CBSE Central 2016]
Ans. (i) According to Henry’s law, p = KH x, i.e., higher the value of KH lower is the solubility of the gas in
the liquid. Therefore, Gas B will have higher value of KH than gas A.
(ii) In non-ideal solution, negative deviation shows the formation of maximum boiling azeotropes.
Q. 12. Derive the relationship between relative lowering of vapour pressure and molar mass of the solute. [CBSE Chennai 2015]
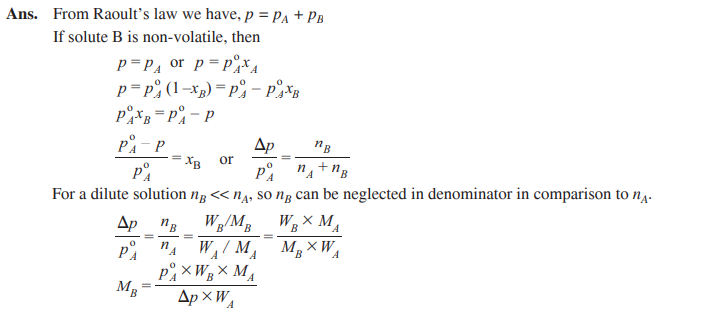
Q. 13. When 1.5 g of a non-volatile solute was dissolved in 90 g of benzene, the boiling point of benzene raised from 353.23 K to 353.93 K. Calculate the molar mass of the solute. (Kb for benzene = 2.52 K kg mol–1) [CBSE Chennai 2015]
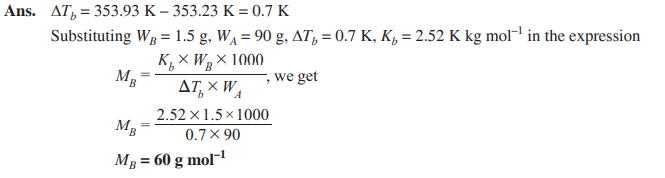
Q. 14. Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180 g mol–1) in 250 g of water. (Kf of water = 1.86 K kg mol–1) [CBSE Delhi 2018]
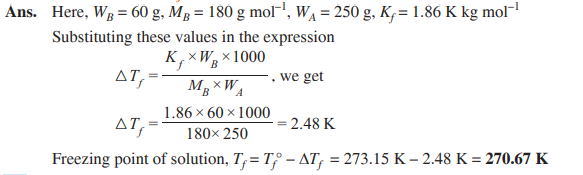
Q. 15. Define the term osmotic pressure. Describe how the molecular mass of a substance can be determined by a method based on measurement of osmotic pressure. [CBSE Delhi 2012]

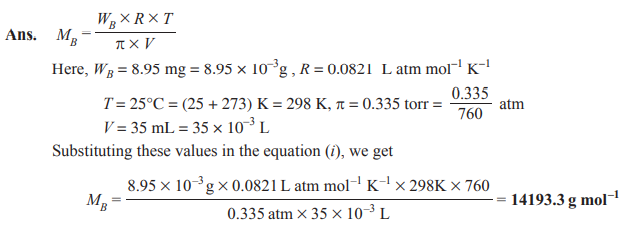
Q. 16. A solution prepared by dissolving 8.95 mg of a gene fragment in 35.0 mL of water has an osmotic pressure of 0.335 torr at 25°C. Assuming that the gene fragment is a non-electrolyte, calculate its molar mass. [CBSE (AI) 2011]
Q. 17. Give reasons: [CBSE 2019 (56/2/1)]
(i) Cooking is faster in pressure cooker than in cooking pan.
(ii) Red Blood Cells (RBC) shrink when placed in saline water but swell in distilled water.
Ans. (i) The use of pressure cooker reduces cooking time because the weight over the lid does not allow the steam to go out. As a result, pressure inside the cooker becomes high. Higher the pressure, higher is the boiling point and faster is the cooking.
(ii) As the concentration of saline solution is higher than the concentration inside the cell. Thus water will move outside the cytoplasm and the cell will shrink while, distilled water is hypotonic, when RBCs are placed in distilled water, water will enter the cell through simple diffusion and lead to cell swelling.
Q. 18. (i) On mixing liquid X and liquid Y, volume of the resulting solution decreases. What type of deviation from Raoult’s law is shown by the resulting solution? What change in temperature would you observe after mixing liquids X and Y?
(ii) What happens when we place the blood cell in water (hypotonic solution)? Give reason. [CBSE Allahabad 2015]
Ans. (i) # The solution will show negative deviation from Raoult’s law.
# Temperature will rise.
(ii) Due to osmosis water enters into the cell and blood cell will swell.
Q. 19. Define the following terms:
(i) Abnormal molar mass
(ii) van’t Hoff factor (i) [CBSE Delhi 2017]
Ans. (i) When the molar mass of a substance determined by using any of the colligative properties comes out to be different than the theoretically expected molar mass, the substance said to show abnormal molar mass.
(ii) van’t Hoff factor (i) gives the extent of association or dissociation of the solute particles in the solution. It may be defined as the ratio of observed colligative property to calculated colligative property.
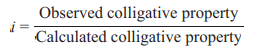
Q. 20. Will the elevation in boiling point be same if 0.1 mol of sodium chloride or 0.1 mol of sugar is dissolved in 1 L of water? Explain. [CBSE Sample Paper 2016]
Ans. No, the elevation in boiling point is not the same. NaCl, being an electrolyte, dissociates almost completely to give Na+ and Cl– ions whereas glucose, being non-electrolyte does not dissociate. Hence, the number of particles in 0.1 M NaCl solution is nearly double than 0.1 M glucose solution. Elevation in boiling point being a colligative property, is therefore, nearly twice for 0.1 M NaCl solution than for 0.1 M glucose solution.
Also See – Very Short Answer Questions Solutions – Chapter 2 CBSE Class 12 Chemistry – Neutron Classes

Pingback: Short Answer Questions – 3 marks – Solutions – Chapter 2 CBSE Class 12 Chemistry - Neutron Classes