A nucleophile is a molecule that forms a bond with its reaction partner (the electrophile) by donating both electrons for that bond. Nucleophiles are Lewis bases.
The word ‘nucleophile’ can be split into two parts, namely nucleus and philos. Philos is the Greek word for ‘love’. Therefore, nucleophiles can be thought of as Nucleus Loving species. These nucleophiles may have either a negative, or a neutral charge.
Below are some examples of nucleophiles.
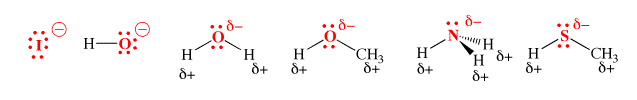
Types of Nucleophiles
Commonly, the following species form good nucleophiles:
- Halogens – the diatomic form of a halogen does not exhibit nucleophilic qualities. However, the anionic form of these halogens are great nucleophiles. An example of this observation is: diatomic iodine (I2) does not act as nucleophile whereas I– is the strongest nucleophile in a polar, protic solvent.
- Carbon – carbon acts as a nucleophile in many organometallic reagents and also in enols. Some examples of compounds wherein carbon acts as a nucleophile include Grignard Reagents, Organolithium Reagents, and n-butyllithium.
- Oxygen – The hydroxide ion is a great example of a nucleophile wherein the electron pair is donated by the oxygen atom. Other examples include alcohols and hydrogen peroxide. It is important to note that no nucleophilic attacks occur during the intermolecular hydrogen bonding that takes place in many compounds containing oxygen and hydrogen.
- Sulphur – due to the large size, the relative ease in its polarization, and the easily accessible lone electron pairs, sulphur has many nucleophilic qualities. Hydrogen sulphide (H2S) is a great example of a nucleophile containing sulphur.
- Nitrogen – Nitrogen is known to form many nucleophiles such as amines, azides, ammonia, and nitrides. Even amides are known to exhibit nucleophilic qualities.
